Designed for – Class VII-X
Time – 1 hour
Objectives
Acidic precipitation is very harmful to the environment and ecology. In this experiment, we explain the increasing ocean acidity and the role of increasing Carbon di Oxide in the atmosphere and the Keeling Curve.
Prerequisites
Before the beginning of the experiment, briefly describe the role of increasing ocean acidity by Carbon di Oxide. Also, a basic idea of pH indicator solution working principle would be good.
Tools required
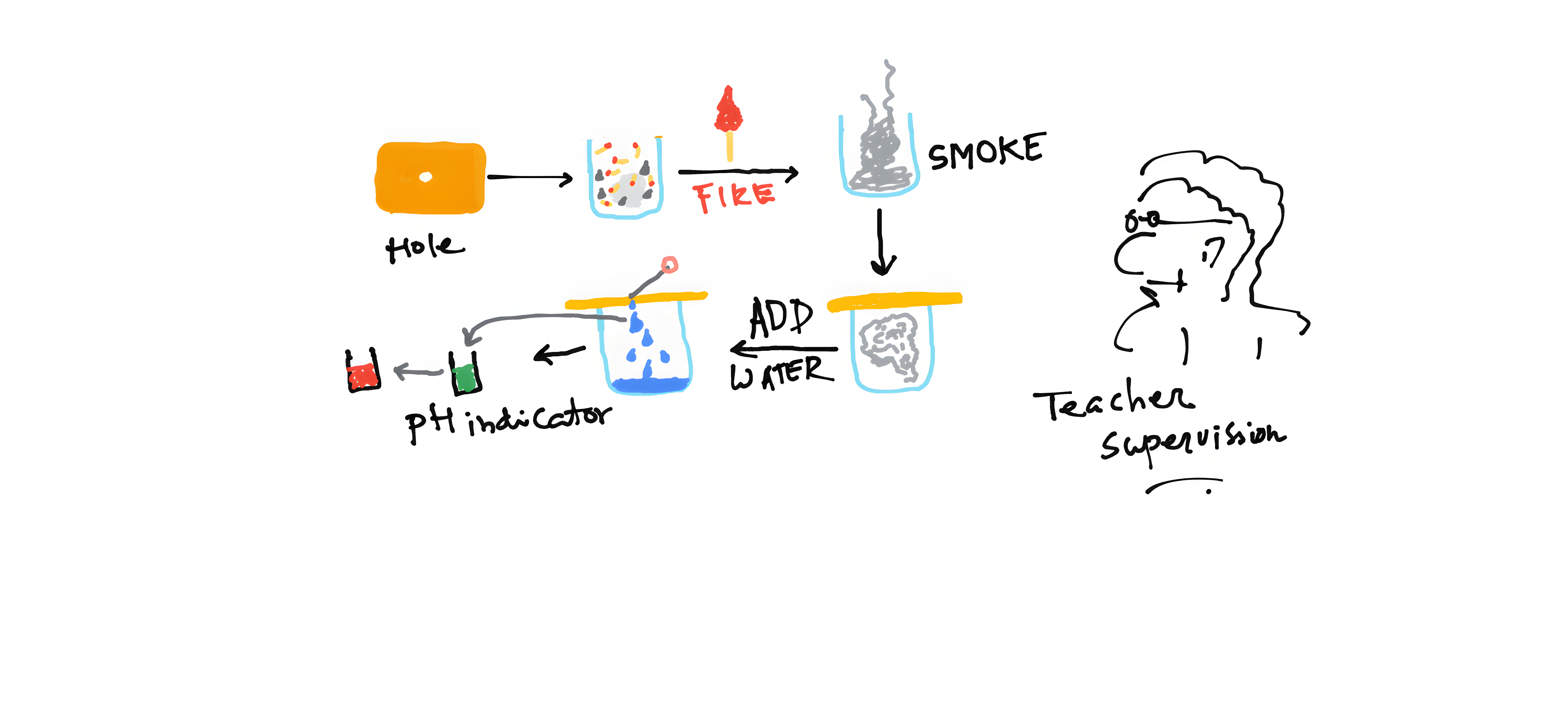
Match stick, coal, cotton balls, 50ml of water, a large glass beaker, a pitch board to cover the beaker, pH change indicator.
 Methods
Methods
The first half of the experiment should be performed by an adult. Make a small hole in the pitch board to add water. Take some matchstick, a little bit of coal and a cotton ball into the glass beaker. Lit fire very carefully, and once the smoke is coming out, close the beaker with the pitch board. Now, add water through the hole and shake the beaker carefully so that some portion of the smoke at least gets solubilized in the water. After 2 minutes, take some water and add it to the pH indicator solution.
Expected results
The color of the pH indicator will be changed after adding the water. The burning creates lots of acidic gas and also CO2, which dissolve in water to make it acidic. Therefore, when it adds to the pH indicator solution, the color change. As the CO2 is increasing in the atmosphere, the ocean (here the pH indicator solution), absorbs more CO2 and becomes more acidic.
Pro-tips
You can perform the same experiment and explain acid rain.