Designed for – Class VI-X
Time – 30 minutes
Objectives
Carbon di Oxide is a major greenhouse gas in the atmosphere which is directly linked to the global rise in temperature. The experiments will help to visualize the relationship between CO2 and temperature.
Prerequisites
Before the beginning of the experiment, briefly describe the history of experiments conducted by Eunice Newton Foote and Tyndal during the 18th century. In addition, how the global CO2 and temperature change, the infamous results from the Mauna Lau observatory should be shown.
Tools required
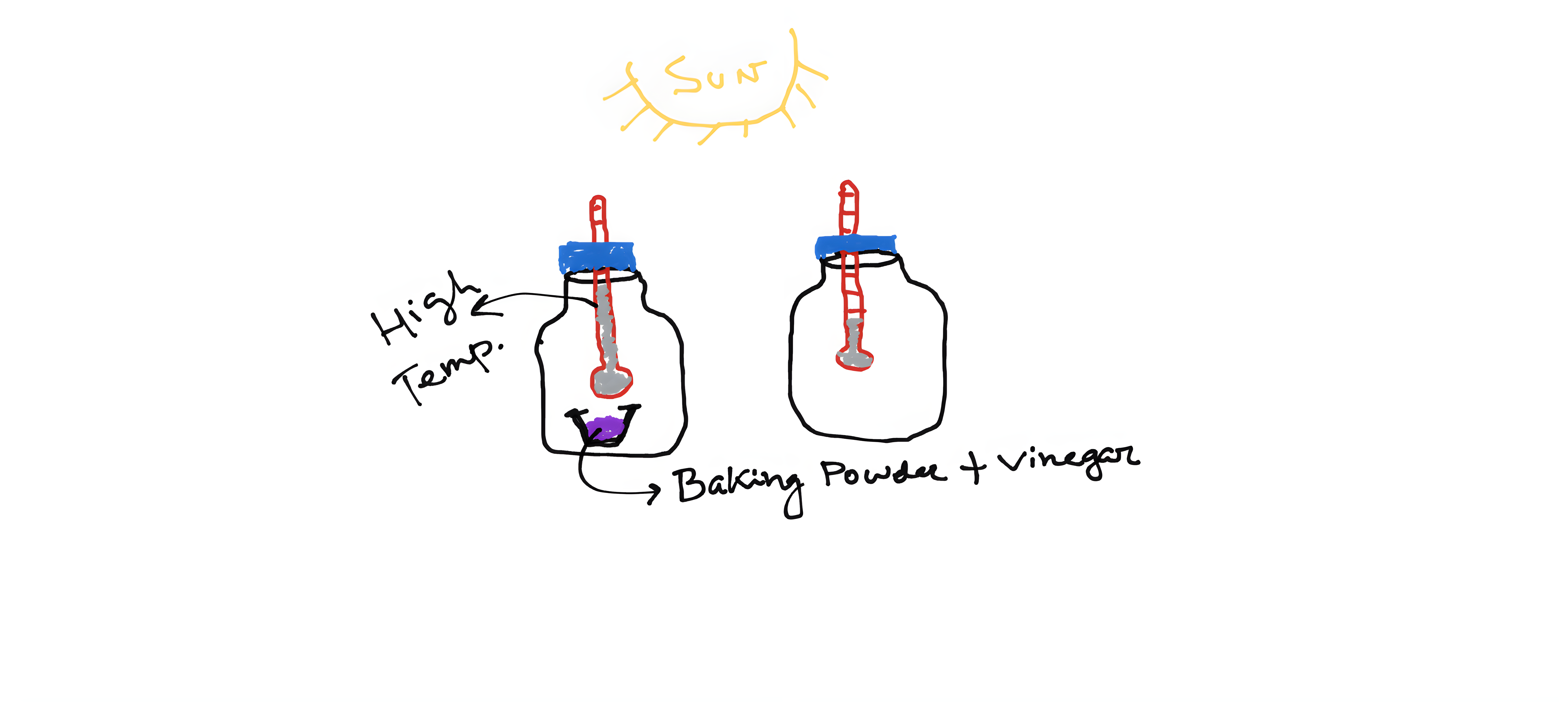
Two glass jars (empty jam bottles work perfectly); two small-size glass thermometers; Plastic warps; a small glass container that goes inside the jar; a tablespoon of baking powder; one cup (~100ml) of vinegar.
Methods
Put one thermometer in each of the glass jars in such a way that the thermometer reading is visible from outside. Now cover the lid of one glass jar with plastic wrap. Now, for the other glass jar, put the small glass container inside. Now add vinegar and baking soda to the small glass container. Effervescence should be visible immediately. Now quickly close the lid of the glass jar using plastic wrap. Now keep these two jars outside in the Sun or under a powerful light (100w bulb). Ask the students to measure the temperature of two glass jars after 5, 10 and 15 minutes.
Expected results
Vinegar and baking soda react to form Carbon di Oxide. The jar with vinegar and baking soda should have a higher CO2 concentration and therefore the temperature in this jar will be higher as compared to the blank jar. The students will measure the temperature of both jars in time intervals and try to see if it changes through time. The average temperature for two jars should be plotted and the instructor can talk about different CO2 sources and global sectoral CO2 concentration.
Pro-tips
The experiments work best on sunny clear days.